NH3(g) + Cl2(g) -> NH4Cl(s)

Mathematics, 18.04.2020 01:43, amison64
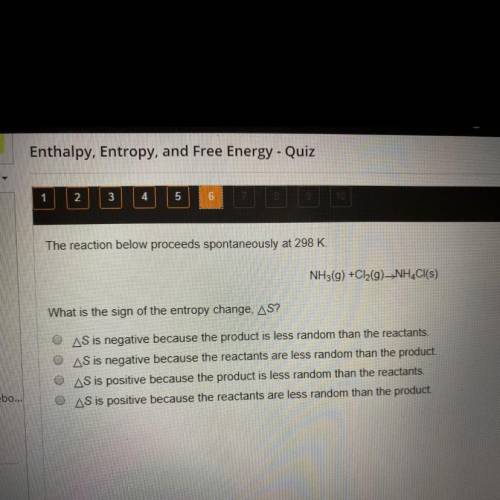
The reaction below proceeds spontaneously at 298 K.
NH3(g) + Cl2(g) -> NH4Cl(s)
What is the sign of the entropy change, delta S?

Answers: 2
Other questions on the subject: Mathematics

Mathematics, 21.06.2019 13:30, gabrielmtrue
Acity plans to build a new rectangular-shaped park. the perimeter of the park will be 940 meters. the width of the park will be 300 meters. what will be the length, in meters, of the new park?
Answers: 1



Mathematics, 21.06.2019 21:10, zahradawkins2007
Identify the initial amount a and the growth factor b in the exponential function. a(x)=680*4.3^x
Answers: 2
Do you know the correct answer?
The reaction below proceeds spontaneously at 298 K.
NH3(g) + Cl2(g) -> NH4Cl(s)
NH3(g) + Cl2(g) -> NH4Cl(s)
Questions in other subjects:

History, 21.01.2021 22:40


Mathematics, 21.01.2021 22:40







Geography, 21.01.2021 22:40







