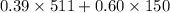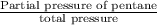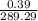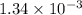Mathematics, 03.03.2020 22:42, upadrastakameswari
Pentane (C5H12) and hexane (C6H14) combine to form an ideal solution. At 258C the vapor pressures of pen- tane and hexane are 511 and 150. torr, respectively. A solution is prepared by mixing 25 mL of pentane (density 5 0.63 g/mL) with 45 mL of hexane (density 5 0.66 g/mL).
(a) What is the vapor pressure of this solution? torr
(b) What is the mole fraction of pentane in the vapor that is in equilibrium with this solution?

Answers: 1
Other questions on the subject: Mathematics

Mathematics, 21.06.2019 13:30, Gladistshiala267
Express the following as a function of a single angle, cos(60) cos(-20) - sin(60) sin(-20)
Answers: 3



Do you know the correct answer?
Pentane (C5H12) and hexane (C6H14) combine to form an ideal solution. At 258C the vapor pressures of...
Questions in other subjects:


Mathematics, 27.01.2021 21:30






Mathematics, 27.01.2021 21:30


Mathematics, 27.01.2021 21:30


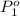 = 511 torr,
= 511 torr, 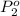 = 150 torr
= 150 torr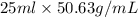
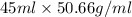
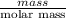
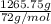
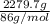
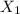 ) =
) = 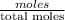
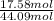
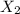 ) =
) = 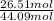
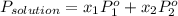
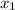 = mole fraction of solution 1
= mole fraction of solution 1
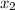 = mole fraction of solution 2
= mole fraction of solution 2