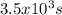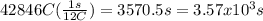Mathematics, 09.10.2019 16:10, erykp17
How many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten alcl3 with an electrical current of 12.0 a? how many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten alcl3 with an electrical current of 12.0 a? 3.57 × 103 1.19 × 103 9.00 2.90 × 105 27.0

Answers: 1
Similar questions

Chemistry, 13.07.2019 20:10, paigemeyers6
Answers: 3

Chemistry, 21.07.2019 14:30, Victor3756
Answers: 1

Physics, 22.07.2019 11:30, itsbabyc
Answers: 1
Do you know the correct answer?
How many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten al...
Questions in other subjects:

English, 16.07.2021 05:50






History, 16.07.2021 05:50

Mathematics, 16.07.2021 05:50

Physics, 16.07.2021 05:50