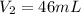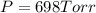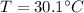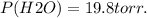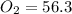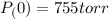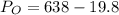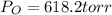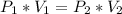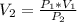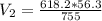Engineering, 20.07.2021 01:00, Wolfgirl2032
Calculate the pressure of dry O2 if the total pressure of O2 generated over water is measured to be 698 Torr and the temperature is 30.1 oC. P(H2O) = 19.8 torr. If the volume of the O2 sample in the question above was 56.3 ml, what volume would the dry O2 occupy at 755 torr (assume the temp was unchanged).

Answers: 1
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, jadeochoa4466
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10, alyssabailey7545
Give heat transfer applications for the following, (i) gas turbines (propulsion) ) gas turbines (power generation). (iii) steam turbines. (iv) combined heat and power (chp). (v) automotive engines
Answers: 1

Engineering, 04.07.2019 18:10, ijohnh14
Shafts are machine elements that are used to a) carry axial loads b) direct shear loads c) transmit power d) rotate at constant speed e) none of the above circular and square shafts subjected to the same torque under the same circum behave a) the same way b) almost the same way
Answers: 2

Engineering, 04.07.2019 18:10, Candi9697
A-mn has a cubic structure with a0 0.8931 nm and a density of 7.47 g/cm3. b-mn has a different cubic structure, with a0 0.6326 nm and a density of 7.26 g/cm3. the atomic weight of manganese is 54.938 g/mol and the atomic radius is 0.112 nm. determine the percent volume change that would occur if a-mn transforms to b-mn.
Answers: 2
Do you know the correct answer?
Calculate the pressure of dry O2 if the total pressure of O2 generated over water is measured to be...
Questions in other subjects:


Mathematics, 03.04.2021 03:50


Mathematics, 03.04.2021 03:50



Mathematics, 03.04.2021 03:50



Mathematics, 03.04.2021 03:50