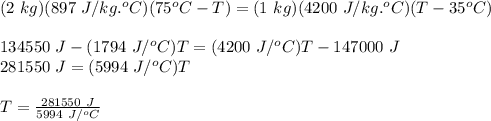Engineering, 17.05.2021 17:50, proxydayz
A 2.00 kg piece of aluminum metal at 75.0 °C is placed in 6.00 liters (= 6.00 kg) of water at 35.0 °C. Determine the final temperature .

Answers: 3
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, lerasteidl
Determine whether or not it is possible to compress air adiabatically from k to 140 kpa and 400 k. what is the entropy change during this process?
Answers: 3

Engineering, 04.07.2019 18:10, Tyrant4life
Draw the engineering stress-strain curve for (a) bcc; (b) fcc metals and mark important points.
Answers: 1

Engineering, 04.07.2019 18:20, safiyabrowne7594
Ahe-xe mixture containing a 0.75 mole fraction of helium is used for cooling electronics in an avionics application. at a temperature of 300 k and atmospheric pressure, calculate the mass fraction of helium and the mass density, molar concentration and molecular weight of the mixture. if the cooling capacity is 10 l, what is the mass of the coolant?
Answers: 3

Engineering, 04.07.2019 18:20, rbgrh9465
An open feedwater heater operates at steady state with liquid entering at inlet 1 with t? = 40°c and pl = 1 .2 mpa. water vapor att2-200°c and p2 = 1.2 mpa enters at inlet 2. saturated liquid water exits with a pressure of pa 1.2 mpa. neglect heat transfer with the surroundings and all kinetic and potential energy effects, determine the mass flow rate of steam at inlet 2 if the mass flow rate of liquid water at inlet 1 is given as 2 kg/s.
Answers: 3
Do you know the correct answer?
A 2.00 kg piece of aluminum metal at 75.0 °C is placed in 6.00 liters (= 6.00 kg) of water at 35.0 °...
Questions in other subjects:


Mathematics, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00

Biology, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00

Biology, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00

History, 12.02.2021 14:00

Biology, 12.02.2021 14:00

History, 12.02.2021 14:00


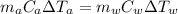
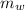 = mass of water = (Density)(Volume) = (1000 kg/m³)(1 L)(0.001 m³/1 L)
= mass of water = (Density)(Volume) = (1000 kg/m³)(1 L)(0.001 m³/1 L)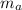 = mass of auminum piece = 2 kg
= mass of auminum piece = 2 kg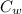 = specific heat capacity of water = 4200 J/kg.°C
= specific heat capacity of water = 4200 J/kg.°C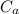 = specific heat capacity of aluminum = 897 J/kg.°C
= specific heat capacity of aluminum = 897 J/kg.°C = Change in Temperature of Water = T - 35°C
= Change in Temperature of Water = T - 35°C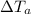 = Change in Temperature of Aluminum Piece = 75°C - T
= Change in Temperature of Aluminum Piece = 75°C - T