
Engineering, 18.03.2021 02:30, madiness05
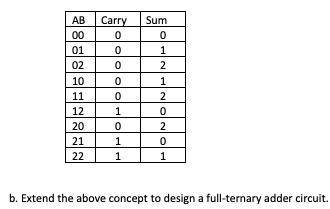
In a ternary number system (i. e., base 3) there are three digits: 0, 1 and 2. The following table defines a ternary half adder. Design a circuit that implements this half adder using binary encoded signals, such that two bits are used for each ternary digit. Let A = a1a0, b = b1b0, and Sum = s1s0; note that Carry is just a binary signal. Use the following encoding: 00 = (0)3, 01 = (1)3, and 10 = (2)3. Minimize the cost of the circuit.
AB Carry Sum
00 0 0
01 0 1
02 0 2
10 0 1
11 0 2
12 1 0
20 0 2
21 1 0
22 1 1
b. Extend the above concept to design a full-ternary adder circuit.


Answers: 3
Other questions on the subject: Engineering


Engineering, 04.07.2019 18:10, abdirahmansoloman
Air is to be cooled in the evaporator section of a refrigerator by passing it over a bank of 0.8-cm-outer-diameter and 0.4-m-long tubes inside which the refrigerant is evaporating at -20°c. air approaches the tube bank in the normal direction at 0°c and 1 atm with a mean velocity of 4 m/s. the tubes are arranged in-line with longitudinal and transverse pitches of sl- st 1.5 cm. there are 30 rows in the flow direction with 15 tubes in each row. determine (a) the refrigeration capacity of this system and (b) pressure drop across the tube bank. evaluate the air properties at an assumed mean temperature of -5°c and 1 atm. is this a good assumption?
Answers: 1

Engineering, 04.07.2019 19:10, jimena15
10 kg of co2 is initially contained at 400 kpa and 300 k. the gas constant for carbon dioxide is 189 j/lkg k) and has a specific heat ratio, k, of 1.289. isentropic expansion then occurs until the pressure is 200 kpa. a) determine the initial volume of co2 in m. b) determine the final temperature in k. c) determine the work done by the system during the expansion kl.
Answers: 2

Engineering, 04.07.2019 19:10, gabrielaperezcz
Air inially occupying a volume of 1 m2 at 100 kpa, 27 c undergoes three internally reversible processes in series. process 1-2 compression to 500 kpa during which pv constant process 2-3 adiabatic expanslon to 100 kpa process 3-1: constant-pressure expansion to 100 kpa (a) calculate the change of entropy for each of the three processes. (b) calculate the heat and work involved in each process. (c) is this cycle a power cycle or refrigeration cycle?
Answers: 3
Do you know the correct answer?
In a ternary number system (i. e., base 3) there are three digits: 0, 1 and 2. The following table d...
Questions in other subjects:




Mathematics, 19.07.2019 14:00


Geography, 19.07.2019 14:00


Chemistry, 19.07.2019 14:00


History, 19.07.2019 14:00






