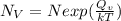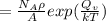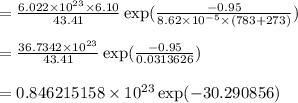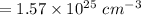Engineering, 27.11.2020 05:30, Maryllen
Calculate the number of vacancies per cubic meter for some metal, M, at 783°C. The energy for vacancy formation is 0.95 eV/atom, while the density and atomic weight for this metal are 6.10 g/cm^3 (at 783°C) and 43.41 g/mol, respectively.

Answers: 2
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, agpraga23ovv65c
Carbon dioxide gas expands isotherm a turbine from 1 mpa, 500 k at 200 kpa. assuming the ideal gas model and neglecting the kinetic and potential energies, determine the change in entropy, heat transfer and work for each kilogram of co2.
Answers: 2


Engineering, 04.07.2019 18:20, safiyabrowne7594
Ahe-xe mixture containing a 0.75 mole fraction of helium is used for cooling electronics in an avionics application. at a temperature of 300 k and atmospheric pressure, calculate the mass fraction of helium and the mass density, molar concentration and molecular weight of the mixture. if the cooling capacity is 10 l, what is the mass of the coolant?
Answers: 3

Engineering, 04.07.2019 18:20, luisgonz5050
Find the kinematic pressure of 160kpa. for air, r-287 j/ kg k. and hair al viscosity of air at a temperature of 50°c and an absolute (10 points) (b) find the dynamic viscosity of air at 110 °c. sutherland constant for air is 111k
Answers: 3
Do you know the correct answer?
Calculate the number of vacancies per cubic meter for some metal, M, at 783°C. The energy for vacanc...
Questions in other subjects:


Physics, 10.03.2020 08:06

Mathematics, 10.03.2020 08:06







English, 10.03.2020 08:06