
Engineering, 16.10.2020 09:01, ally6440
Thermodynamics deals with the macroscopic properties of materials. Scientists can make quantitative predictions about these macroscopic properties by thinking on a microscopic scale. Kinetic theory and statistical mechanics provide a way to relate molecular models to thermodynamics. Predicting the heat capacities of gases at a constant volume from the number of degrees of freedom of a gas molecule is one example of the predictive power of molecular models. The molar specific heat Cv of a gas at a constant volume is the quantity of energy required to raise the temperature T of one mole of gas by one degree while the volume remains the same. Mathematically, Cv=1nΔEthΔT, where n is the number of moles of gas, ΔEth is the change in internal (or thermal) energy, and ΔT is the change in temperature. Kinetic theory tells us that the temperature of a gas is directly proportional to the total kinetic energy of the molecules in the gas. The equipartition theorem says that each degree of freedom of a molecule has an average energy equal to 12kBT, where kB is Boltzmann's constant 1.38×10^−23J/K. When summed over the entire gas, this gives 12nRT, where R=8.314Jmol⋅K is the ideal gas constant, for each molecular degree of freedom.
Required:
a. Using the equipartition theorem, determine the molar specific heat, Cv , of a gas in which each molecule has s degrees of freedom. Express your answer in terms of R and s.
b. Given the molar specific heat Cv of a gas at constant volume, you can determine the number of degrees of freedom s that are energetically accessible. For example, at room temperature cis-2-butene, C4H8 , has molar specific heat Cv=70.6Jmol⋅K . How many degrees of freedom of cis-2-butene are energetically accessible?

Answers: 2
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, soreese02
An ideal otto cycle with air as the working fluid has a compression ratio of 8. the minimum and maximum temperatures in the cycle are 300 k and 1340 k. use constant specific heats at room temperature to determine (a) the amount of heat transferred to the air during the heat- addition kj/kg, (b) the thermal efficiency, and (c) the thermal efficiency of a carnot cycle ope limits. process, in rating between the same temperature
Answers: 2

Engineering, 04.07.2019 18:10, tobyhollingsworth178
Which from the following instrument is commonly used to detect the high pitch butzing sound in bearings? [clo4] a)-digital ultrasonic meter b)-infrared camera c)-spectroscopic d)-vibrometer
Answers: 2

Engineering, 04.07.2019 18:20, annette211pdd8v9
For a gate width of 2 m into the paper, determine the force required to hold the gate abc at its location.
Answers: 1

Engineering, 04.07.2019 18:20, rhussein6452
Wiy doeres rere okhn a pump whon working betwon the same pressure range?
Answers: 2
Do you know the correct answer?
Thermodynamics deals with the macroscopic properties of materials. Scientists can make quantitative...
Questions in other subjects:

Biology, 21.08.2021 23:10





Business, 21.08.2021 23:10

English, 21.08.2021 23:10


Geography, 21.08.2021 23:10


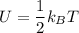






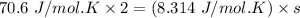

 17
17



