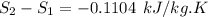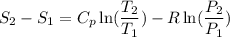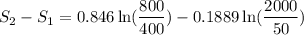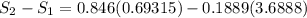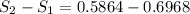Engineering, 14.07.2020 14:01, sandram74691
Carbon dioxide initially at 50 kPa, 400 K, undergoes a process in a closed system until its pressure and temperature are 2 MPa and 800 K, respectively. Assuming an ideal gas behaviour, find the entropy change of the carbon dioxide by assuming that the specific heats are constant. For the gas, take Cp = 0.846 kJ/kg. K and R = 0.1889 kJ/kg. K

Answers: 3
Other questions on the subject: Engineering

Engineering, 03.07.2019 15:10, breannaasmith1122
Two flowing streams of argon gas are adiabatically mixed to form a single flow/stream. one stream is 1.5 kg/s at 400 kpa and 200 c while the second stream is 2kg/s at 500 kpa and 100 ? . it is stated that the exit state of the mixed single flow of argon gas is 150 c and 300 kpa. assuming there is no work output or input during the mixing process, does this process violate either the first or the second law or both? explain and state all your assumptions.
Answers: 1

Engineering, 04.07.2019 03:10, lauriepdx17
What precautions should you take to prevent injuries when dealing with heavy loads?
Answers: 1


Engineering, 04.07.2019 19:20, justicehernandez
Acarnot refrigerator operates in a room in which the temperature is 21°c and of power when operating. if the food compartment of the refrigerator is consumes 3 kw to be maintained at 2°c, determine (a) the coefficient of performance of the cycle and (b) the rate of heat removal from the food compartment. refrigerator cycle that has a higher coefficient of performance than that of the discussed (e) is it possible to develop a carn ot refrigerator, operating between the same temperature limits? explain
Answers: 2
Do you know the correct answer?
Carbon dioxide initially at 50 kPa, 400 K, undergoes a process in a closed system until its pressure...
Questions in other subjects:

Mathematics, 09.01.2020 23:31

History, 09.01.2020 23:31


Mathematics, 09.01.2020 23:31




Chemistry, 09.01.2020 23:31


Mathematics, 09.01.2020 23:31