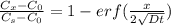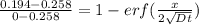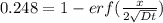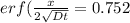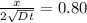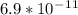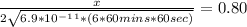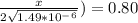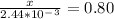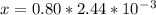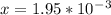Engineering, 18.06.2020 15:57, zara76
An iron-carbon alloy initially containing 0.258 wt% C is exposed to an oxygen-rich and virtually carbon-free atmosphere at 1120°C. Under these circumstances the carbon diffuses from the alloy and reacts at the surface with the oxygen in the atmosphere; that is, the carbon concentration at the surface position is maintained essentially at 0.0 wt% C. At what position will the carbon concentration be 0.194 wt% after a 6 h treatment? The value of D at 1120°C is 6.9 × 10-11 m2/s.

Answers: 2
Other questions on the subject: Engineering




Engineering, 04.07.2019 19:10, LadyHolmes67
Apressure vessel with an r/t 20 cannot be treated as thin walled vessel. a)-trune b)- false
Answers: 3
Do you know the correct answer?
An iron-carbon alloy initially containing 0.258 wt% C is exposed to an oxygen-rich and virtually car...
Questions in other subjects:





Geography, 06.02.2021 02:10


Mathematics, 06.02.2021 02:10

Health, 06.02.2021 02:10

Mathematics, 06.02.2021 02:10