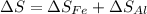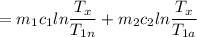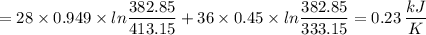Engineering, 18.06.2020 04:57, justinbailey96
An aluminum block weighing 28 kg initially at 140°C is brought into contact with a block of iron weighing 36 kg at 60°C in an insulated enclosure. Determine the final equilibrium temperature and the total entropy change for this process. The specific heat of aluminum at 400 K is cp = 0.949 kJ/kg·K. The specific heat of iron at room temperature is cp = 0.45 kJ/kg·K.

Answers: 3
Other questions on the subject: Engineering

Engineering, 04.07.2019 16:10, TheOriginalMeyah
An electrical motor raises a 50kg load at a construct velencity .calculate the power of the motor, if it takes 40sec to raise the load through a height of 24m(take g =9.8n/g)
Answers: 2

Engineering, 04.07.2019 18:10, xboxdude06
Slip occurs via two partial dislocations because of (a) the shorter path of the partial dislocation lines; (b) the lower energy state through partial dislocations; (c) the charge balance.
Answers: 1

Engineering, 04.07.2019 18:10, Larkinlover703
Items are similar to the free issue items, but their access is limited. (clo5) a)-bin stock items free issue b)-bin stock controlled issue c)-critical or insurance spares d)-rebuildable spares e)-consumables
Answers: 1

Engineering, 04.07.2019 18:10, samanthabutryn
Which one from below is not one of the reasons of planning failures? (clo3) a)-planner is careless. b-planner spend less time in the field but more time on the desk c)-planner is not qualified d)-planner does not have sufficient time to properly plan
Answers: 3
Do you know the correct answer?
An aluminum block weighing 28 kg initially at 140°C is brought into contact with a block of iron wei...
Questions in other subjects:

Chemistry, 12.10.2020 04:01

English, 12.10.2020 04:01

Mathematics, 12.10.2020 04:01

English, 12.10.2020 04:01

Chemistry, 12.10.2020 04:01


History, 12.10.2020 04:01