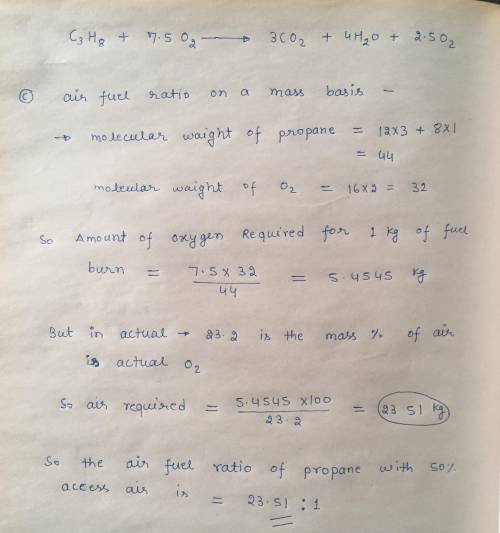Engineering, 06.05.2020 02:09, dorindaramirez0531
Propane gas C3H8 enters a combustion chamber operating at steady state condition at 1 bar, 25ºC and is burned with 150% theoretical air that enters the combustion chamber at the same state. The products leave the combustion chamber at 1 bar and 1200 K.
a) Write down the balanced chemical equation for the complete combustion.
b) Write down the balanced actual combustion reaction and calculate c) the air fuel ratio on a mass basis, d) the dew point temperature of the products in ºC, e) the heat transfer in terms of kJ/kmol of fuel.

Answers: 2
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:20, luisgonz5050
Find the kinematic pressure of 160kpa. for air, r-287 j/ kg k. and hair al viscosity of air at a temperature of 50°c and an absolute (10 points) (b) find the dynamic viscosity of air at 110 °c. sutherland constant for air is 111k
Answers: 3

Engineering, 04.07.2019 18:20, samantha636
Avolume of 2.65 m3 of air in a rigid, insulated container fitted with a paddle wheel is initially at 264 k, 5.6 bar. the air receives 432 kj by work from the paddle wheel. assuming the ideal gas model with cv = 0.71 kj/kg • k, determine for the air the amount of entropy produced, in kj/k
Answers: 2


Engineering, 04.07.2019 19:20, dndndndnxmnc
The process in which the system pressure remain constant is called a)-isobaric b)-isochoric c)-isolated d)-isothermal
Answers: 3
Do you know the correct answer?
Propane gas C3H8 enters a combustion chamber operating at steady state condition at 1 bar, 25ºC and...
Questions in other subjects:

Social Studies, 06.09.2021 17:40


Mathematics, 06.09.2021 17:40



Chemistry, 06.09.2021 17:40




Mathematics, 06.09.2021 17:40