
Engineering, 08.04.2020 04:36, gorbyalexis
A closed, rigid tank is filled with a gas modeled as an ideal gas, initially at 60°C and a gage pressure of 300 kPa. The gas is heated, and the gage pressure at the final state is 600 kPa. The local atmospheric pressure is 1 atm. Determine the final temperature, in °C.

Answers: 1
Other questions on the subject: Engineering

Engineering, 03.07.2019 14:10, cowgyrlup124
Explain the difference laminar and turbulent flow. explain it with the shear stress and the velocity profiles.
Answers: 1

Engineering, 03.07.2019 23:20, abbz13
Two technicians are discussing the intake air temperature (iat) sensor. technician a says that the computer uses the iat sensor as a backup to the engine coolant temperature (ect) sensor. technician b says that the powertrain control module (pcm) will subtract the calculated amount of fuel if the air measures hot. who is correct
Answers: 3

Engineering, 04.07.2019 18:10, genyjoannerubiera
Assuming compressible flow of air and that the measurements are done at flagstaff a pitot static tube that gives the difference of total and static pressure measures 0.35 m of mercury. what is the velocity of air? assume the temperature to be 300k. (submit your excel or matlab calculation sheet)
Answers: 1

Engineering, 04.07.2019 18:20, hayleymckee
Steam enters a converging nozzle at 3.0 mpa and 500°c with a at 1.8 mpa. for a nozzle exit area of 32 cm2, determine the exit velocity, mass flow rate, and exit mach number if the nozzle: negligible velocity, and it exits (a) is isentropic (b) has an efficiency of 94 percent
Answers: 2
Do you know the correct answer?
A closed, rigid tank is filled with a gas modeled as an ideal gas, initially at 60°C and a gage pres...
Questions in other subjects:

Mathematics, 24.06.2019 20:30

English, 24.06.2019 20:30


Mathematics, 24.06.2019 20:30


Mathematics, 24.06.2019 20:30



Social Studies, 24.06.2019 20:30

Mathematics, 24.06.2019 20:30


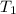 = 60 °C = 333.15 K Initial temperature
= 60 °C = 333.15 K Initial temperature 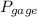 = 300 kPa , gage initial pressure ,
= 300 kPa , gage initial pressure ,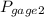 = 600 kPa , gage final pressure ,
= 600 kPa , gage final pressure ,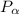 = 101.325 kPa
= 101.325 kPa 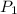 =
= 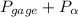
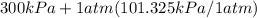
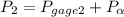
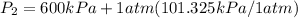
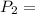 600. 299 kPa
600. 299 kPa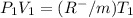 this is equation one
this is equation one 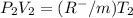 this is equation two
this is equation two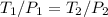
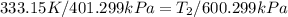
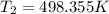 = 225.205 °C
= 225.205 °C



