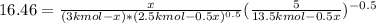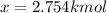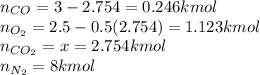Engineering, 02.04.2020 03:10, maiahfogel1351
An equilibrium mixture of 3 kmol of CO, 2.5 kmol of O2, and 8 kmol of N2 is heated to 2600 K at a pressure of 5 atm. Determine the equilibrium composition of the mixture for these conditions.

Answers: 1
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, jadeochoa4466
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10, meganwintergirl
Afour cylinder four-stroke in-line engine has a stroke of 160mm, connecting rod length of 150mm, a reciprocating mass of 3kg and its firing order is 1-3-4-2. the spacing between cylinders is 100mm. i. show that the engine is in balance with regard to the primary inertia forces and primary 3. a and secondary inertia couples. li determine the out of balance secondary inertia force ii. propose ways of balancing this out of balance force and discuss the challenges that will arise
Answers: 3

Engineering, 04.07.2019 18:10, lillygrl100
For the closed feedwater heater below, feedwater enters state 3 at a pressure of 2000 psia and temperature of 420 °f at a rate of ix10 ibhr. the feedwat extracted steam enters state 1 at a pressure of 1000 psia and enthalpy of 1500 btu/lbm. the extracted er leaves at an enthalpy of 528.7 btu/lbm steam leaves as a saturated liquid. (16) a) determine the mass flow rate of the extraction steam used to heat the feedwater (10) b) determine the terminal temperature difference of the closed feedwater heater
Answers: 3

Engineering, 04.07.2019 18:10, johnthienann58
Thermal stresses are developed in a metal when its a) initial temperature is changed b) final temperature is changed c) density is changed d) thermal deformation is prevented e) expansion is prevented f) contraction is prevented
Answers: 2
Do you know the correct answer?
An equilibrium mixture of 3 kmol of CO, 2.5 kmol of O2, and 8 kmol of N2 is heated to 2600 K at a pr...
Questions in other subjects:








Biology, 20.07.2019 19:00

History, 20.07.2019 19:00

Business, 20.07.2019 19:00


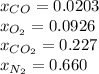
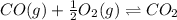
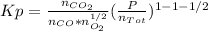
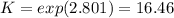
 the equilibrium goes:
the equilibrium goes: