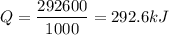Engineering, 30.03.2020 21:25, coolkitty35
Find the amount of energy (Q) required to raise the temperature of the water in kilo joules (KJ).
Given mass of water m = 2kg = 2000g.
Temperature difference ΔΤ = T2 – T1 = 60 °C – 25 °C = 35 °C .
Specific heat of water = 4.18 J/g* °C .
Therefore, Q =
kilo joules.

Answers: 3
Other questions on the subject: Engineering

Engineering, 03.07.2019 15:10, EmilySerna
Heat is added to a piston-cylinder device filled with 2 kg of air to raise its temperature 400 c from an initial temperature of t1 27 cand pressure of pi 1 mpa. the process is isobaric process. find a)-the final pressure p2 b)-the heat transfer to the air.
Answers: 1

Engineering, 03.07.2019 23:20, abbz13
Two technicians are discussing the intake air temperature (iat) sensor. technician a says that the computer uses the iat sensor as a backup to the engine coolant temperature (ect) sensor. technician b says that the powertrain control module (pcm) will subtract the calculated amount of fuel if the air measures hot. who is correct
Answers: 3

Engineering, 04.07.2019 18:10, wyattlb97
Water at the rate of 1 kg/s is forced through a tube with a 2.5 cm inner diameter. the inlet water temperature is 15°c, and the outlet water temperature is 50°c. the tube wall temperature is 14°c higher than the local water temperature all along the length of the tube. what is the length of the tube?
Answers: 3

Engineering, 04.07.2019 18:20, alexis9263
Have a greater impact on maintenance productivity than any other support group. (clo5) a)-the top management b)-inventory and purchasing c)-sub-contracting d)-cmms
Answers: 2
Do you know the correct answer?
Find the amount of energy (Q) required to raise the temperature of the water in kilo joules (KJ).
Questions in other subjects:

History, 13.10.2020 16:01

Health, 13.10.2020 16:01

Biology, 13.10.2020 16:01

Mathematics, 13.10.2020 16:01


English, 13.10.2020 16:01


Mathematics, 13.10.2020 16:01

English, 13.10.2020 16:01