
Engineering, 30.03.2020 16:59, paulitaaustin
The reverse water-gas shift (RWGS) reaction is an equimolar reaction between CO2 and H2 to form CO and H2O. Assume CO2 associatively adsorbs to the surface, while H2 dissociatively adsorbs. These adsorption steps are followed by reversible formation of formate (COOH*) and slow dissociation of formate into gaseous CO and adsorbed OH. The adsorbed OH is then removed as gaseous H2O via a hydrogenation step.
a) Using the details of the mechanism listed above, write out the elementary steps for the RWGS reaction.
b) Derive a rate law for the RWGS reaction consistent with the above assumptions and mechanism from (i).
c) Under what conditions is the RWGS reaction first order in CO2?

Answers: 3
Other questions on the subject: Engineering

Engineering, 03.07.2019 15:10, theamandawhite
Ahouse has the following electrical appliance usage (1) single 40w lamp used for 4 hours per day (2) single 60w fan used for 12 hours per day (3) single 200w refrigerator that runs 24 hours per day with compressor run 12 hours and off 12 hours find the solar power inverter size in watt with correction factor of 1.25.
Answers: 1


Engineering, 04.07.2019 18:10, alyssabailey7545
Give heat transfer applications for the following, (i) gas turbines (propulsion) ) gas turbines (power generation). (iii) steam turbines. (iv) combined heat and power (chp). (v) automotive engines
Answers: 1

Engineering, 04.07.2019 18:10, qwertylol12345
Different types of steels contain different elements that alter the characteristics of the steel. for each of the following elements, explain what the element does when alloyed with steel.
Answers: 2
Do you know the correct answer?
The reverse water-gas shift (RWGS) reaction is an equimolar reaction between CO2 and H2 to form CO a...
Questions in other subjects:


Mathematics, 26.01.2020 02:31

Social Studies, 26.01.2020 02:31



Law, 26.01.2020 02:31


Biology, 26.01.2020 02:31


Mathematics, 26.01.2020 02:31


![Rate = \frac{k_{1}k_{4} }{k_{3}+ 2k_{4} } [H_{2} ]](/tpl/images/0570/4445/89073.png)
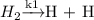 (Fast process)
(Fast process)![\[ CO_{2} + H\mathrel{\mathop{\rightleftarrows}^{\mathrm{k2}}_{\mathrm{k3}}}COOH \]](/tpl/images/0570/4445/0ee99.png) (Fast Process)
(Fast Process)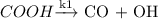 (Slow process)
(Slow process)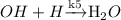 (Fast process)
(Fast process)![\frac{d[COOH]}{dt} = 0](/tpl/images/0570/4445/953ae.png)
![k_{2} [CO_{2} ][H] = k_{3} [COOH] k_{4} [COOH]\\](/tpl/images/0570/4445/d3edf.png)
![[COOH] = \frac{k_{2} [CO_{2} ][H]}{k_{3}k_{4} } \\](/tpl/images/0570/4445/2668d.png)
![\frac{d[H]}{dt} = 0](/tpl/images/0570/4445/8807a.png)
![k_{1}[H_{2}] = k_{2}[CO_{2} [H]+k_{5} [ OH][H]](/tpl/images/0570/4445/93949.png)
![[H]= \frac{k_{1}[H_{2}] }{k_{5}[OH] +k_{2}[CO_{2}]}\\](/tpl/images/0570/4445/c726a.png)
![\frac{d[OH]}{dt} = 0](/tpl/images/0570/4445/d1756.png)
![k_{4} [COOH] = k_{5} [OH][H]\\\k[OH] = \frac{k_{4} [COOH]}{k_{5} H}\\](/tpl/images/0570/4445/833a9.png)
![Rate = k_{4} [COOH]\\](/tpl/images/0570/4445/d9edf.png)
![Rate = k_{4} \frac{k_{2} [CO_{2} ][H]}{k_{3}k_{4} }\\Rate = k_{4} \frac{k_{2}[CO_{2}]\frac{k_{1}[H_{2}] }{k_{5}[OH] +k_{2}[CO_{2}]} }{k_{3}k_{4}}\\Rate = k_{4} \frac{k_{2}[CO_{2}]\frac{k_{1}[H_{2}] }{k_{5}\frac{k_{4}COOH }{k_{5}H } +k_{2}[CO_{2}]} }{k_{3}k_{4}}](/tpl/images/0570/4445/8876a.png)




