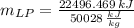Engineering, 13.03.2020 05:03, Arealbot
What mass of LP gas is necessary to heat 1.4 L of water from room temperature (25.0 ∘C) to boiling (100.0 ∘C)? Assume that during heating, 16% of the heat emitted by the LP gas combustion goes to heat the water. The rest is lost as heat to the surroundings. Express your answer using two significant figures.

Answers: 2
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, settasav9641
Abrake has a normal braking torque of 2.8 kip in and heat-dissipating cast-iron surfaces whose mass is 40 lbm. suppose a load is brought to rest in 8.0 s from an initial angular speed of 1600 rev/min using the normal braking torque; estimate the temperature rise of the heat dissipating surfaces.
Answers: 3


Engineering, 04.07.2019 18:20, annette211pdd8v9
For a gate width of 2 m into the paper, determine the force required to hold the gate abc at its location.
Answers: 1
Do you know the correct answer?
What mass of LP gas is necessary to heat 1.4 L of water from room temperature (25.0 ∘C) to boiling (...
Questions in other subjects:

History, 12.07.2019 23:30

Mathematics, 12.07.2019 23:30

Mathematics, 12.07.2019 23:30

Mathematics, 12.07.2019 23:30


English, 12.07.2019 23:30





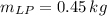
![Q_{water} = (1.4\,L)\cdot(\frac{1\,m^{3}}{1000\,L} )\cdot (1000\,\frac{kg}{m^{3}} )\cdot [(4.187\,\frac{kJ}{kg\cdot ^{\textdegree}C} )\cdot (100^{\textdegree}C-25^{\textdegree}C)+2257\,\frac{kJ}{kg}]](/tpl/images/0546/2617/21482.png)
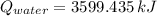
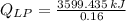
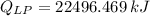
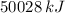 . Then, the required mass is:
. Then, the required mass is: