
Engineering, 06.03.2020 23:42, andybiersack154
Air expands adiabatically through a nozzle from a negligible initial velocity to a final velocity of 300 m/s, what is the temperature drop of the air, if air is assumed to be an ideal gas for which CP = (7/2)R?

Answers: 3
Other questions on the subject: Engineering

Engineering, 03.07.2019 15:10, theamandawhite
Ahouse has the following electrical appliance usage (1) single 40w lamp used for 4 hours per day (2) single 60w fan used for 12 hours per day (3) single 200w refrigerator that runs 24 hours per day with compressor run 12 hours and off 12 hours find the solar power inverter size in watt with correction factor of 1.25.
Answers: 1

Engineering, 04.07.2019 18:10, jadeochoa4466
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10, winterblanco
The higher the astm grain-size number, the coarser the grain is. a)-true b)-false
Answers: 3

Engineering, 04.07.2019 18:20, samantha636
Avolume of 2.65 m3 of air in a rigid, insulated container fitted with a paddle wheel is initially at 264 k, 5.6 bar. the air receives 432 kj by work from the paddle wheel. assuming the ideal gas model with cv = 0.71 kj/kg • k, determine for the air the amount of entropy produced, in kj/k
Answers: 2
Do you know the correct answer?
Air expands adiabatically through a nozzle from a negligible initial velocity to a final velocity of...
Questions in other subjects:

Chemistry, 28.04.2021 19:10




English, 28.04.2021 19:10

Mathematics, 28.04.2021 19:10

Mathematics, 28.04.2021 19:10


Computers and Technology, 28.04.2021 19:10

Chemistry, 28.04.2021 19:10


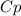 ×ΔT
×ΔT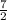 ×8.314
×8.314



