
Engineering, 28.02.2020 04:02, paytonthalacker
Air, modeled as an ideal gas, is compressed at steady state from 1 bar, 300 K, to 5 bar, 500 K, with 30 kW of power input. Heat transfer occurs at a rate of 4.000 kW from the air to cooling water circulating in a water jacket enclosing the compressor. Neglecting kinetic and potential energy effects, determine the mass flow rate of the air, in kg/s.

Answers: 3
Other questions on the subject: Engineering

Engineering, 03.07.2019 15:10, EmilySerna
Heat is added to a piston-cylinder device filled with 2 kg of air to raise its temperature 400 c from an initial temperature of t1 27 cand pressure of pi 1 mpa. the process is isobaric process. find a)-the final pressure p2 b)-the heat transfer to the air.
Answers: 1


Engineering, 04.07.2019 18:10, genyjoannerubiera
Assuming compressible flow of air and that the measurements are done at flagstaff a pitot static tube that gives the difference of total and static pressure measures 0.35 m of mercury. what is the velocity of air? assume the temperature to be 300k. (submit your excel or matlab calculation sheet)
Answers: 1

Engineering, 04.07.2019 18:10, QueenLife4869
Awall of 0.5m thickness is to be constructed from a material which has average thermal conductivity of 1.4 w/mk. the wall is to be insulated with a material having an average thermal conductivity of 0.35 w/mk so that heat loss per square meter shall not exceed 1450 w. assume inner wall surface temperature of 1200°c and outer surface temperature of the insulation to be 15°c. calculate the thickness of insulation required.
Answers: 3
Do you know the correct answer?
Air, modeled as an ideal gas, is compressed at steady state from 1 bar, 300 K, to 5 bar, 500 K, with...
Questions in other subjects:




Arts, 12.11.2020 22:50


Mathematics, 12.11.2020 22:50



Mathematics, 12.11.2020 22:50

Health, 12.11.2020 22:50


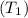 and pressure into the system are 300 K and 1 bar respectively. The outlet temperature
and pressure into the system are 300 K and 1 bar respectively. The outlet temperature 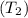 and pressure
and pressure 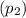 are 500 K and 5 bar respectively. The heat transfer rate
are 500 K and 5 bar respectively. The heat transfer rate 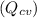 is 30 kW and the power input
is 30 kW and the power input 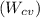 is 4000 kW.
is 4000 kW.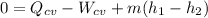
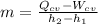
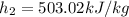 ,
,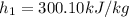 ,
, 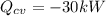 , and
, and 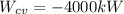 . Therefore:
. Therefore:



