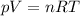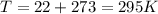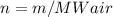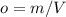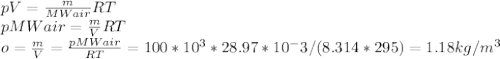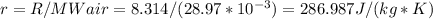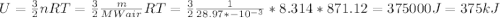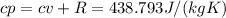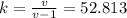Engineering, 21.02.2020 21:01, tesie
Determine the unknown quantity, assuming air behaves as an ideal gas. R¯ = 8.314 kJ kmol−1 K −1 . MWair = 28.97 kg kmol−1 . If needed, average the specific heats between the beginning and ending temperatures.1. At I bar and 22 C, determine o and r. 2. At 10 bar and v 0.25 m3 kg 1, determine T and u. 3. At 600 K, determine cp, Cu, k, and v

Answers: 3
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, leomessifanboy678
The filament of an incandescent lamp has a temperature of 2000k. calculate the fraction of radiation emitted in the visible light band if the filament is approximated as blackbody
Answers: 2

Engineering, 04.07.2019 18:10, mamasbug4285
An air compression refrigeration system is to have an air pressure of 100 psia in the brine tank and an allowable air temperature increase of 60°f for standard vapor compression cycle temperatures of 77 f entering the expansion cylinder and 14 f entering the compression cylinder, calculate the coefficient of performance a. 2.5 b 3.3 c. 4.0 d. 5.0
Answers: 3

Engineering, 04.07.2019 18:20, sanchez626
Aheavily insulated piston-cylinder device contains 0.02 m3 of steam at 300 kpa and 200 °c. 1.2 mpa. d this process. team is now compressed in a reversible manner to a pressure of etermine the entropy change and the work done on the steam during this process
Answers: 1

Engineering, 04.07.2019 18:20, cdyshaylia55
Water vapor initially at 10 bar and 400 °c is contained within a piston-cylinder assembly. the water lost heat to the surrounding according to isochoric (iso-volumetric) process until its temperature is 150 °c. the water is then condensed isothermally to saturated liquid. for the water as a system, calculate the work in kj/kg
Answers: 2
Do you know the correct answer?
Determine the unknown quantity, assuming air behaves as an ideal gas. R¯ = 8.314 kJ kmol−1 K −1 . MW...
Questions in other subjects:


Mathematics, 21.04.2020 22:09



History, 21.04.2020 22:09



Mathematics, 21.04.2020 22:09

Computers and Technology, 21.04.2020 22:09

Mathematics, 21.04.2020 22:09