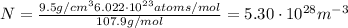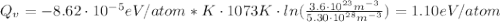Engineering, 20.02.2020 04:31, oof40
Calculate the energy for vacancy formation in silver, given that the equilibrium number of vacanciesat 800°C (1073 K) is 3.6 × 1023m–3. The atomic weight and density (at 800°C) for silver are, respectively, 107.9 g/mol and 9.5 g/cm3. (hint: see example problem in Lecture 9for part of the solution)

Answers: 2
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, redrosesxx
Water at 55c flows across a flat plate whose surface temperature is held constant at 95c. if the temperature gradient at the plate's surface for a given value of x is 18 c/mm, find a) local heat transfer coefficient. b) heat flux
Answers: 3

Engineering, 04.07.2019 18:10, agpraga23ovv65c
Carbon dioxide gas expands isotherm a turbine from 1 mpa, 500 k at 200 kpa. assuming the ideal gas model and neglecting the kinetic and potential energies, determine the change in entropy, heat transfer and work for each kilogram of co2.
Answers: 2

Engineering, 04.07.2019 18:10, xboxdude06
Slip occurs via two partial dislocations because of (a) the shorter path of the partial dislocation lines; (b) the lower energy state through partial dislocations; (c) the charge balance.
Answers: 1
Do you know the correct answer?
Calculate the energy for vacancy formation in silver, given that the equilibrium number of vacancies...
Questions in other subjects:



English, 19.05.2021 22:30

Mathematics, 19.05.2021 22:30

Biology, 19.05.2021 22:30

Business, 19.05.2021 22:30


English, 19.05.2021 22:30


Mathematics, 19.05.2021 22:30


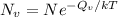
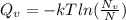 (1)
(1) 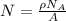
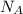 : is the Avogadro constant = 6.022x10²³ atoms/mol, and A: is the atomic weight = 107.9 g/mol.
: is the Avogadro constant = 6.022x10²³ atoms/mol, and A: is the atomic weight = 107.9 g/mol.