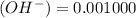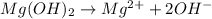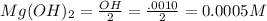Engineering, 13.02.2020 23:54, Arm2005
A water initially contains 40 mg · L−1 of Mg2+. The pH of the water is increased until the concentration of hydroxide ion (OH−) is 0.001000 M. What is the concentration of magnesium ion in this water at this pH? Give your answer in milligrams per liter. Assume that the temperature of the solution is 25◦

Answers: 1
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, viicborella
Steel is coated with a thin layer of ceramic to protect against corrosion. what do you expect to happen to the coating when the temperature of the steel is increased significantly? explain.
Answers: 1

Engineering, 04.07.2019 18:20, 1230bering
Select any two (2) areas of applications of chain-drive. (clo4) a)-permanent lubrication necessary b)-hydraulic forklift truck operation c)-rigging and heavy moving materials d)-relatively high maintenance costs e)-costlier than belt drives
Answers: 2

Engineering, 04.07.2019 19:10, phantomlizz3233
In general, how do thermosetting plastics compare to thermoplastics in mechanical and physical properties?
Answers: 3

Engineering, 04.07.2019 19:10, jennymares
Abarometer contains mercury with a density of 13600 kg/m3. atmospheric conditions are 95.8 kpa and 20 °c at 20 °c, the vapor pressure of the mercury is 0.000173 kpa. the column of mercury will rise to a height of most nearly. select one: a)- 0.38 m b)- 0.82 m c)- 0.48 m d)- 0.72 m
Answers: 1
Do you know the correct answer?
A water initially contains 40 mg · L−1 of Mg2+. The pH of the water is increased until the concentra...
Questions in other subjects:



Mathematics, 29.12.2020 23:10

Business, 29.12.2020 23:10




English, 29.12.2020 23:10

Mathematics, 29.12.2020 23:10