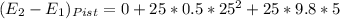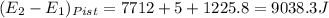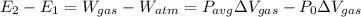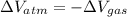Engineering, 02.11.2019 06:31, dannyo6680
A25 kg piston is above a gas in a long vertical cylinder. now the piston is released from rest and accelerates up in the cylinder reaching the end 5 m higher at a velocity of 25 m/s. the gas pressure drops during the process, so the average is 600 kpa with an outside atmosphere at 100 kpa. neglect the change in gas kinetic and potential energy, and find the needed change in the gas volume.

Answers: 1
Other questions on the subject: Engineering

Engineering, 03.07.2019 14:10, makaylashrout77
Amass of 1.5 kg of air at 120 kpa and 24°c is contained in a gas-tight, frictionless piston-cylinder device. the air is now compressed to a final pressure of 720 kpa. during the process, heat is transferred from the air such that the temperature inside the cylinder remains constant. calculate the boundary work input during this process.
Answers: 2

Engineering, 04.07.2019 18:10, viicborella
Steel is coated with a thin layer of ceramic to protect against corrosion. what do you expect to happen to the coating when the temperature of the steel is increased significantly? explain.
Answers: 1

Engineering, 04.07.2019 18:10, lerasteidl
Determine whether or not it is possible to compress air adiabatically from k to 140 kpa and 400 k. what is the entropy change during this process?
Answers: 3

Engineering, 04.07.2019 18:10, agpraga23ovv65c
Carbon dioxide gas expands isotherm a turbine from 1 mpa, 500 k at 200 kpa. assuming the ideal gas model and neglecting the kinetic and potential energies, determine the change in entropy, heat transfer and work for each kilogram of co2.
Answers: 2
Do you know the correct answer?
A25 kg piston is above a gas in a long vertical cylinder. now the piston is released from rest and a...
Questions in other subjects:



Mathematics, 30.11.2020 05:10

Mathematics, 30.11.2020 05:10

History, 30.11.2020 05:10

Mathematics, 30.11.2020 05:10


Mathematics, 30.11.2020 05:10


Mathematics, 30.11.2020 05:10


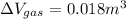
![(E_2-E_1)_{Pist}=m(u_2-u_1)+m[\frac{1}{2}V^2_2-0]+mg(h_2-0)](/tpl/images/0356/6304/418c2.png)