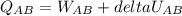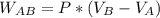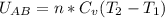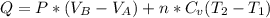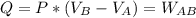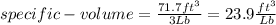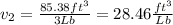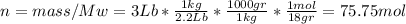Engineering, 19.10.2019 02:10, isalih7256
Asystem consisting of 3 lb of water vapor in a piston–cylinder assembly, initially at 350°f and a volume of 71.7 ft3, is expanded in a constant‐pressure process to a volume of 85.38 ft3. the system then is compressed isothermally to a final volume of 28.2 ft3. during the isothermal compression, energy transfer by work into the system is 72 btu. kinetic and potential energy effects are negligible. determine the heat transfer, in btu, for each process.

Answers: 3
Other questions on the subject: Engineering

Engineering, 04.07.2019 03:10, lauriepdx17
What precautions should you take to prevent injuries when dealing with heavy loads?
Answers: 1

Engineering, 04.07.2019 18:10, yasminothman02
An air conditioning system consist of a 5 cm diameter pipe, operating at a pressure of 200 kpa. the air initially enters the pipe at 15°c with a velocity of 20 m/s and relative humidity of 80%. if the heat supply throughout the process is 960 w, determine the relative humidity and the temperature at the outlet
Answers: 3

Engineering, 04.07.2019 18:10, Larkinlover703
Items are similar to the free issue items, but their access is limited. (clo5) a)-bin stock items free issue b)-bin stock controlled issue c)-critical or insurance spares d)-rebuildable spares e)-consumables
Answers: 1

Engineering, 04.07.2019 18:20, jessie8022
Apiston-cylinder device contains 0.1 m3 of liquid water and 0.9 m3 of water vapor in equilibrium at 800 kpa. heat is transferred at constant pressure until the temperature of water reaches 350 °c. determine (a) the quality of water at the initial state (b) the work associated with this process, (c) the heat associated with this process.
Answers: 2
Do you know the correct answer?
Asystem consisting of 3 lb of water vapor in a piston–cylinder assembly, initially at 350°f and a vo...
Questions in other subjects:




Mathematics, 29.10.2020 23:10

Spanish, 29.10.2020 23:10

Mathematics, 29.10.2020 23:10

Mathematics, 29.10.2020 23:10

Computers and Technology, 29.10.2020 23:10