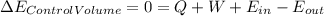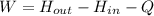Engineering, 01.10.2019 06:00, erikpaynee8622
An ideal gas at a flow rate of 10m3/min enters a compressor at 25 °c and 1 bar. it leaves at 1 mpa. during this process, heat is dissipated to the surroundings at a rate of 2100 w. you may that the surroundings to be at a constant temperature of 25 °c. the heat capacity of the ideal gas is given by c_p/r=2.00+0.040t where t in in units of k. answer the following assuming that the process is reversible, calculate the temperature at the compressor outlet. calculate the minimum power required to compress the gas. if the compressor efficiency is 70%, calculate the actual power needed. calculate the actual final temperature.

Answers: 2
Similar questions

Engineering, 18.09.2019 02:30, athenawalcroff
Answers: 2

Physics, 06.10.2019 04:01, brucewayne390
Answers: 2
Do you know the correct answer?
An ideal gas at a flow rate of 10m3/min enters a compressor at 25 °c and 1 bar. it leaves at 1 mpa....
Questions in other subjects:


Mathematics, 01.09.2021 04:10

Mathematics, 01.09.2021 04:10

Mathematics, 01.09.2021 04:10




Mathematics, 01.09.2021 04:10


Mathematics, 01.09.2021 04:10


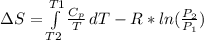
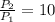 . Then, the expression can be simplified to:
. Then, the expression can be simplified to:![8.3144*[2*ln(\frac{T_{2}}{T_{1}} ) + 0.04*(T_{2} - T_{1})] - 8.3144*ln(10) = 0](/tpl/images/0278/9391/239b0.png)

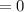 Assuming that the process in in steady state:
Assuming that the process in in steady state: