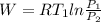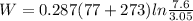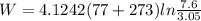Engineering, 29.09.2019 02:30, nockturnal1993
Agas is compressed isothermally in a piston-cylinder assembly from 7.6 bar, 77 °c to 3.05 bar. determine the work and heat transfer, kj/kg, for the following gases. a) air b) hydrogen

Answers: 3
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, alyssabailey7545
Give heat transfer applications for the following, (i) gas turbines (propulsion) ) gas turbines (power generation). (iii) steam turbines. (iv) combined heat and power (chp). (v) automotive engines
Answers: 1

Engineering, 04.07.2019 18:10, lillygrl100
For the closed feedwater heater below, feedwater enters state 3 at a pressure of 2000 psia and temperature of 420 °f at a rate of ix10 ibhr. the feedwat extracted steam enters state 1 at a pressure of 1000 psia and enthalpy of 1500 btu/lbm. the extracted er leaves at an enthalpy of 528.7 btu/lbm steam leaves as a saturated liquid. (16) a) determine the mass flow rate of the extraction steam used to heat the feedwater (10) b) determine the terminal temperature difference of the closed feedwater heater
Answers: 3


Engineering, 04.07.2019 19:10, juneham
Estimate the change in specific internal energy au and specific enthalpy h from inlet to outlet for ethylene glycol (a liquid) flowing through each of the following devices: (a) a heat exchanger where the glycol temperature increases from 20 °c to 80 °c; (b) a pump operating at about 25 °c and increasing the glycol pressure from 100 kpa to 8 mpa.
Answers: 2
Do you know the correct answer?
Agas is compressed isothermally in a piston-cylinder assembly from 7.6 bar, 77 °c to 3.05 bar. deter...
Questions in other subjects:



Mathematics, 10.01.2020 09:31



Mathematics, 10.01.2020 09:31

History, 10.01.2020 09:31


Mathematics, 10.01.2020 09:31

Mathematics, 10.01.2020 09:31