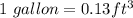Engineering, 23.09.2019 14:30, Jackierose2004
Ignoring any losses, estimate how much energy (in units of btu) is required to raise the temperature of water in a 90-gallon hot-water tank from 60°f to 110°f. the specific heat of water is approximated as a constant, whose value is 0.999 btu/·lbmr at the average temperature of (60 + 110)/2 = 85ºf. in fact, c remains constant at 0.999 btu/lbm·r (to three digits) from 60ºf to 110ºf. for this same temperature range, the density varies from 62.36 lbm/ft3 at 60ºf to 61.86 lbm/ft3 at 110ºf. we approximate the density as remaining constant, whose value is 62.17 lbm/ft3 at the average temperature of 85ºf.

Answers: 1
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, danksans7011
The mass flow rate of the fluid remains constant in all steady flow process. a)- true b)- false
Answers: 1

Engineering, 04.07.2019 18:10, tjeffers90028
Refrigerant 134a enters an insulated compressor operating at steady state as saturated vapor at -26°c with a volumetric flow rate of 0.18 m3/s. refrigerant exits at 9 bar, 70°c. changes in kinetic and potential energy from inlet to exit can be ignored. determine the volumetric flow rate at the exit, in m3/s, and the compressor power, in kw.
Answers: 1

Engineering, 04.07.2019 18:10, caitlynnpatton1208
Water in a partially filled large tank is to be supplied to the roof top, which is 8 m above the water level in the tank, through a 2.2-cm-internal-diameter pipe by maintaining a constant air pressure of 300 kpa (gage) in the tank. if the head loss in the piping is 2 m of water, determine the discharge rate of the supply of water to the roof top in liters per second.
Answers: 3

Engineering, 04.07.2019 19:20, zoebtharpe
Heat transfer by is the fastest mode of heat transfer that requires no intervening medium. a)-conduction b)-convection c)-radiation d)-conduction and convection
Answers: 1
Do you know the correct answer?
Ignoring any losses, estimate how much energy (in units of btu) is required to raise the temperature...
Questions in other subjects:


Mathematics, 17.09.2019 09:50







Mathematics, 17.09.2019 09:50

History, 17.09.2019 09:50