Engineering, 13.09.2019 22:20, hahHcjckk
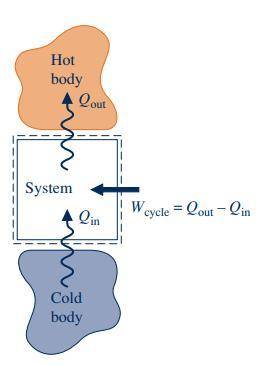
Aheat pump has a work input of 2 kw and provides 7 kw of net heat transfer to heat a house. the system is steady, and there are no other work or heat interactions. is this a violation of the first law of thermodynamics? select one: a. yes b. no

Answers: 2
Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, jadeochoa4466
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10, lerasteidl
Determine whether or not it is possible to compress air adiabatically from k to 140 kpa and 400 k. what is the entropy change during this process?
Answers: 3

Engineering, 04.07.2019 18:10, genyjoannerubiera
Assuming compressible flow of air and that the measurements are done at flagstaff a pitot static tube that gives the difference of total and static pressure measures 0.35 m of mercury. what is the velocity of air? assume the temperature to be 300k. (submit your excel or matlab calculation sheet)
Answers: 1

Engineering, 04.07.2019 18:20, Doogsterr
For each of the following process: a) sketch the p-v diagram, b)sketch t-s diagram, c) sketch t-v diagram, d) sketch the boundary work on one of the diagrams (a, b or c) and e) sketch the reversible heat transfer on one of the diagrams (a, b or c): 1- isobaric process from compressed liquid to superheated vapor 2- isothermal process from compressed liquid to superheated vapor 3- isentropic process from compressed liquid to superheated vapor
Answers: 3
Do you know the correct answer?
Aheat pump has a work input of 2 kw and provides 7 kw of net heat transfer to heat a house. the syst...
Questions in other subjects:


Mathematics, 10.12.2019 00:31

Mathematics, 10.12.2019 00:31

History, 10.12.2019 00:31

Mathematics, 10.12.2019 00:31

Social Studies, 10.12.2019 00:31

Mathematics, 10.12.2019 00:31