
Engineering, 02.09.2019 19:10, msdevdev777
Indicate whether the following statements are true or false for an isothermal process: (a) q=t(∆s). (b) ∆u=0.(c) the entropy change of the system is always zero. (d) the total entropy change of the system and the surroundings is always zero. (e) the entropy change of the surroundings is negative. (f) q=w.

Answers: 3
Other questions on the subject: Engineering

Engineering, 03.07.2019 14:10, bryneosburn
Line joining liquid phase with liquid and solid phase mixture is known as: a) liquidus b) solidus c) tie line d) none of the mentioned
Answers: 2

Engineering, 03.07.2019 15:10, theamandawhite
Ahouse has the following electrical appliance usage (1) single 40w lamp used for 4 hours per day (2) single 60w fan used for 12 hours per day (3) single 200w refrigerator that runs 24 hours per day with compressor run 12 hours and off 12 hours find the solar power inverter size in watt with correction factor of 1.25.
Answers: 1

Engineering, 04.07.2019 03:10, lauriepdx17
What precautions should you take to prevent injuries when dealing with heavy loads?
Answers: 1

Engineering, 04.07.2019 18:10, dval1146
You are making beer. the first step is filling the glass carboy with the liquid wort. the internal diameter of the carboy is 15 in., and you wish to fill it up to a depth of 2 ft. if your wort is drawn from the kettle using a siphon process that flows at 3 gpm, how long will it take to fill?
Answers: 1
Do you know the correct answer?
Indicate whether the following statements are true or false for an isothermal process: (a) q=t(∆s)....
Questions in other subjects:




Mathematics, 01.03.2021 23:20


English, 01.03.2021 23:20


English, 01.03.2021 23:20



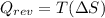
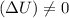 , like in answer B. One case where this is true is in the reversible isothermal expansion (or compression) of an ideal gas.
, like in answer B. One case where this is true is in the reversible isothermal expansion (or compression) of an ideal gas.




