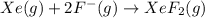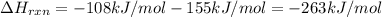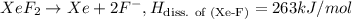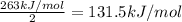Chemistry, 21.07.2019 07:30, Jessieileen
The enthalpy of formation of xef2(g) is –108 kj mol–1 and the bond dissociation enthalpy of the f–f bond is 155 kj mol–1 . what is the average bond dissociation enthalpy of a xe–f bond

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 10:10, Kennethabrown09
In a covalent bond, two atoms are held together by the attraction between . the number of covalent bonds that an atom can form depends on the number of in the atom.
Answers: 2

Chemistry, 23.06.2019 11:40, brendonvernon8
Which of the following would have the lowest average kinetic energy
Answers: 1

Chemistry, 23.06.2019 19:30, NeverEndingCycle
Where do the numbers in a stoichiometry mole ratio come from
Answers: 1

Chemistry, 23.06.2019 20:30, Willywill15
If 4.88 grams of zn react with 5.03 grams of s8 to produce 6.02 grams of zns, what are the theoretical yield and percent yield of this reaction? be sure to show the work that you did to solve this problem. unbalanced equation: zn + s8 yields zns
Answers: 3
Do you know the correct answer?
The enthalpy of formation of xef2(g) is –108 kj mol–1 and the bond dissociation enthalpy of the f–f...
Questions in other subjects:


English, 11.11.2019 17:31


Mathematics, 11.11.2019 17:31



Mathematics, 11.11.2019 17:31

Mathematics, 11.11.2019 17:31


Physics, 11.11.2019 17:31


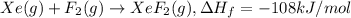 ..(1)
..(1)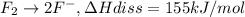 ..(2)
..(2)