
Chemistry, 21.07.2019 08:00, romyknight
Compared to a solution with a ph value of 7 a solution with a thousand times greater hydronium ion concentration has a ph value of what

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, sophiapknight
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 21.06.2019 23:30, 23gordns
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉mo re accurate estimates can be made with the van der waals equationí‘ťí‘ť=í‘›í‘›í‘›í‘›í‘›í‘›í‘ ‰í‘‰â’푛푛푟푟â’푞푞푛푛2í‘ ‰í‘‰2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 04:40, marknjenbennetp3j1v1
Listen base your answer to the question on the information below. propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below. c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol. what is the correct structural formula for a molecule of methanethiol
Answers: 3

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1
Do you know the correct answer?
Compared to a solution with a ph value of 7 a solution with a thousand times greater hydronium ion c...
Questions in other subjects:



Mathematics, 12.03.2021 19:40

Social Studies, 12.03.2021 19:40

Mathematics, 12.03.2021 19:40




Biology, 12.03.2021 19:40

Mathematics, 12.03.2021 19:40


![\rm pH=-\;log\;[H_3O^+]](/tpl/images/0114/8938/91a5a.png)
![\rm pH=\;-log\;[H_3O^+]\\\\ 7=\;-\;log[H_3O^+]\\\\ H_3O^+=10^-^7\;M](/tpl/images/0114/8938/03f6f.png)
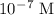 . The concentration of new solution will be:
. The concentration of new solution will be: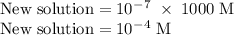
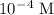 . The pH of the solution has been given as:
. The pH of the solution has been given as:![\rm pH=-log\;[H_3O^+]\\ pH=-log\;[10^-^4]\\ pH=4](/tpl/images/0114/8938/e7183.png)




