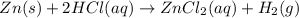Answers: 2
Similar questions

Chemistry, 04.07.2019 00:30, haddockc2689
Answers: 1

Biology, 13.07.2019 22:00, akerner
Answers: 2

Chemistry, 02.08.2019 22:30, 10040813
Answers: 1
Do you know the correct answer?
Given the reaction: zn(s) + 2hcl(aq) → zncl2(aq) + h2(g) the oxidation number of zn(s) increases be...
Questions in other subjects:

Business, 10.12.2020 08:20

Chemistry, 10.12.2020 08:20


Mathematics, 10.12.2020 08:20

Mathematics, 10.12.2020 08:20

Social Studies, 10.12.2020 08:20

English, 10.12.2020 08:20

Mathematics, 10.12.2020 08:20


Mathematics, 10.12.2020 08:20