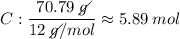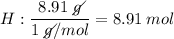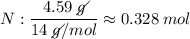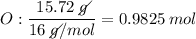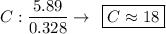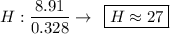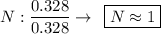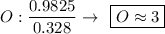Determine the empirical formula for a compound that is 70.79% carbon, 8.91% hydrogen, 4.59% nitrogen, and 15.72% oxygen. determine the empirical formula for a compound that is 70.79 carbon, 8.91 hydrogen, 4.59 nitrogen, and 15.72 oxygen. c18h27no2 c18h27no3 c17h27no3 c17h26no3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:00, bryn2433
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 03:30, antoinetteee03
Name atleast 3 type of energy associated with the microwave
Answers: 1

Chemistry, 23.06.2019 09:30, MendesArmy333
Northern was a learned a fairly cold climate caused by see one from the atlantic ocean, but se was real and tends to be much warmer, sonia look good causes difference. a. cool wins cannot blow across a leg into the south mountains, b. prevent cold air from blowing over into south sea, c.the south is at much higher elevation, so it is closer to the sun, d. suppose early and has a sperience a drastic climate change in the past few years.
Answers: 1

Chemistry, 23.06.2019 11:00, randyg0531
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2
Do you know the correct answer?
Determine the empirical formula for a compound that is 70.79% carbon, 8.91% hydrogen, 4.59% nitrogen...
Questions in other subjects:



Mathematics, 03.09.2020 17:01



Mathematics, 03.09.2020 17:01


Biology, 03.09.2020 17:01