
Agas contained in a steel tank has a pressure of 1.5 atm at a temperature of 320 k. what will be the gas pressure when the temperature changes to 450 k? a gas contained in a steel tank has a pressure of 1.5 atm at a temperature of 320 k. what will be the gas pressure when the temperature changes to 450 k? 1.5 atm 0.47 atm 0.94 atm 2.1 atm 1.1 atm

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
Do you know the correct answer?
Agas contained in a steel tank has a pressure of 1.5 atm at a temperature of 320 k. what will be the...
Questions in other subjects:


World Languages, 26.07.2019 09:30



Mathematics, 26.07.2019 09:30




History, 26.07.2019 09:30

Social Studies, 26.07.2019 09:30


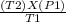
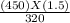 = 2.1 atm
= 2.1 atm



