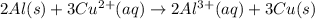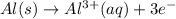Chemistry, 21.07.2019 18:30, theeflyguy5
Given the balanced ionic equation representing a reaction: 2al(s) + 3cu2+(aq) → 2al3+(aq) + 3cu(s)which half-reaction represents the reduction that occurs? al → al3+ +3eal3+ +3e → alcu→ cu2+ +2ecu2+ + 2e → cu

Answers: 2
Similar questions


Chemistry, 02.09.2019 06:50, jazminpratt0311
Answers: 2


Chemistry, 29.09.2019 03:30, rubyr9975
Answers: 1
Do you know the correct answer?
Given the balanced ionic equation representing a reaction: 2al(s) + 3cu2+(aq) → 2al3+(aq) + 3cu(s)w...
Questions in other subjects:

Geography, 15.01.2020 05:31




Mathematics, 15.01.2020 05:31

Biology, 15.01.2020 05:31

English, 15.01.2020 05:31

Mathematics, 15.01.2020 05:31

Mathematics, 15.01.2020 05:31

Mathematics, 15.01.2020 05:31