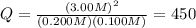Chemistry, 21.07.2019 23:00, falldownguyss
This system has an equilibrium constant of 50.5 at 448°c: h2(g) + i2(g) ↔ 2hi(g). what is the reaction quotient, q, for this system when [h2] = 0.200 m, [i2] = 0.100 m, and [hi] = 3.00 m?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Do you know the correct answer?
This system has an equilibrium constant of 50.5 at 448°c: h2(g) + i2(g) ↔ 2hi(g). what is the react...
Questions in other subjects:



Mathematics, 05.11.2020 19:30


Mathematics, 05.11.2020 19:30

Mathematics, 05.11.2020 19:30


Advanced Placement (AP), 05.11.2020 19:30



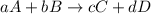
![Q=\frac{[C]^{c}[D]^{d}}{[A]^{a}[B]^{b}}](/tpl/images/0117/2709/a6d96.png)
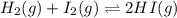
![Q=\frac{[HI]^{2}}{[H_{2}][I_{2}]}](/tpl/images/0117/2709/db0f2.png)