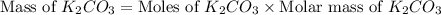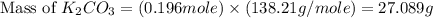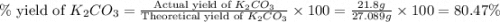Chemistry, 21.07.2019 23:00, asuhdude57
)determine the theoretical yield and the percent yield if 21.8 g of k2co3 is produced from reacting 27.9 g ko2 with 29.0 l of co2 (at stp). the molar mass of ko2 = 71.10 g/mol and k2co3 = 138.21 g/mol.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 09:20, weridness80
Which of the following occurs along coasts during the day?
Answers: 3


Chemistry, 23.06.2019 12:50, vinniemccray70
What is the daughter nucleus produced a. when 217(at) undergoes alpha decay? b. when 103(mo) undergoes beta decay? c. when 188(hg) undergoes positron emission?
Answers: 1
Do you know the correct answer?
)determine the theoretical yield and the percent yield if 21.8 g of k2co3 is produced from reacting...
Questions in other subjects:

Mathematics, 09.04.2020 17:27

Biology, 09.04.2020 17:27


Mathematics, 09.04.2020 17:28

Biology, 09.04.2020 17:28






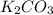 = 27.089 g
= 27.089 g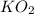 = 27.9 g
= 27.9 g
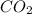 = 29.0 L (At STP)
= 29.0 L (At STP)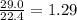 mole of
mole of 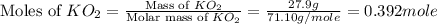
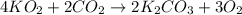
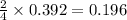 moles of
moles of