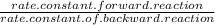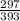Chemistry, 21.07.2019 23:00, ashtynbursiaga
He rate constant for the forward reaction, k1, is 297 l·mol–1·min–1 and the rate constant for the reverse reaction, k–1, is 393 l·mol–1·min–1 at a given temperature. the activation energy for the forward reaction is 42.1 kj·mol–1, while the activation energy for the reverse reaction is 22.1 kj·mol–1. determine the equilibrium constant, k, of this reaction.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
Do you know the correct answer?
He rate constant for the forward reaction, k1, is 297 l·mol–1·min–1 and the rate constant for the re...
Questions in other subjects:

Mathematics, 10.06.2021 08:50

Computers and Technology, 10.06.2021 08:50


Chemistry, 10.06.2021 08:50

Mathematics, 10.06.2021 08:50


Mathematics, 10.06.2021 08:50

Biology, 10.06.2021 08:50

Business, 10.06.2021 08:50

Biology, 10.06.2021 08:50