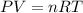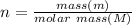Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:50, limelight11
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1

Chemistry, 21.06.2019 20:50, britotellerialuis
Evaluate this exponential expression,8. (2 + 3)2 – 42
Answers: 3

Chemistry, 22.06.2019 04:30, coryoddoc3685
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1
Do you know the correct answer?
Asample of gas at 301 kelvin and 1.5 atmospheres has a density of 3.90 g/l. what is the molar mass o...
Questions in other subjects:

Mathematics, 12.10.2019 15:30

Health, 12.10.2019 15:30




Mathematics, 12.10.2019 15:30


Biology, 12.10.2019 15:30

Chemistry, 12.10.2019 15:30