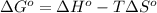Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, zitterkoph
Which of the following mining methods disrupts the sea floor?
Answers: 1

Chemistry, 22.06.2019 00:30, VictoriaRose520
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
Do you know the correct answer?
Acetylene, c2h2, has a standard enthalpy of formation, δh° = 226.7 kj/mol, and a standard entropy ch...
Questions in other subjects:

Social Studies, 06.05.2020 04:32

Computers and Technology, 06.05.2020 04:32



Mathematics, 06.05.2020 04:32

History, 06.05.2020 04:32

Mathematics, 06.05.2020 04:32


English, 06.05.2020 04:32


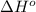 = 226.7 kJ/K mol,
= 226.7 kJ/K mol,  = 58.8 J/mol =
= 58.8 J/mol = 
 = (25 + 273) K = 298 K
= (25 + 273) K = 298 K ) as follows.
) as follows.