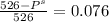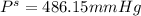Chemistry, 22.07.2019 11:30, ericasolis2614
What is the vapor pressure of a 45.0 % solution of glucose, c6h12o6, at 90.0°c, given that the vapor pressure of pure water at that temperature is 526 mm hg?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 00:30, emilylizbeth12334
Which of the following best describes technology a. something created for only scientists to use b. the method of thinking that scientists use. c. the application of engineering to create useful products. c. a scientific idea
Answers: 1
Do you know the correct answer?
What is the vapor pressure of a 45.0 % solution of glucose, c6h12o6, at 90.0°c, given that the vapor...
Questions in other subjects:

Mathematics, 25.04.2020 01:17

Mathematics, 25.04.2020 01:17




Mathematics, 25.04.2020 01:18

Mathematics, 25.04.2020 01:18

Biology, 25.04.2020 01:18

English, 25.04.2020 01:18

Mathematics, 25.04.2020 01:18


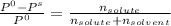 = 0.25 / (0.25 + 3.05)
= 0.25 / (0.25 + 3.05)