
How long must a constant current of 50.0 a be passed through an electrolytic cell containing aqueous cu2+ ions to produce 5.00 moles of copper metal? how long must a constant current of 50.0 a be passed through an electrolytic cell containing aqueous cu2+ ions to produce 5.00 moles of copper metal? 5.36 hours 2.68 hours 0.373 hours 0.187 hours?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, connienash95
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1
Do you know the correct answer?
How long must a constant current of 50.0 a be passed through an electrolytic cell containing aqueous...
Questions in other subjects:


Mathematics, 17.11.2019 08:31

Mathematics, 17.11.2019 08:31


Chemistry, 17.11.2019 08:31

Mathematics, 17.11.2019 08:31

Health, 17.11.2019 08:31



Social Studies, 17.11.2019 08:31





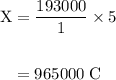

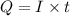
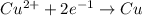
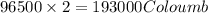 of electricity deposits 1 mole of Cu.
of electricity deposits 1 mole of Cu.
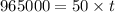
 (1 hour=3600sec)
(1 hour=3600sec)




