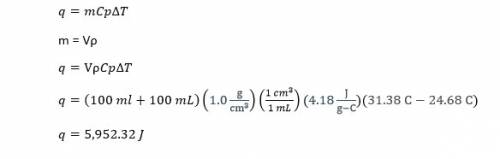In a coffee-cup calorimeter, 100.0 ml of 1.0 m naoh and 100.0 ml of 1.0 m hcl are mixed. both solutions were originally at 24.68c. after the reaction, the final temperature is 31.38c. assuming that all the solutions have a density of 1.0 g/cm3 and a specific heat capacity of 4.18 j/8c ? g, calculate the enthalpy change for the neutralization of hcl by naoh

Answers: 1
Similar questions

Chemistry, 24.06.2019 07:00, mashejaj
Answers: 1

Chemistry, 15.07.2019 17:30, edfrank6278
Answers: 1

Chemistry, 02.08.2019 20:20, alondrachon
Answers: 2
Do you know the correct answer?
In a coffee-cup calorimeter, 100.0 ml of 1.0 m naoh and 100.0 ml of 1.0 m hcl are mixed. both soluti...
Questions in other subjects:






Mathematics, 28.09.2021 19:10




History, 28.09.2021 19:10