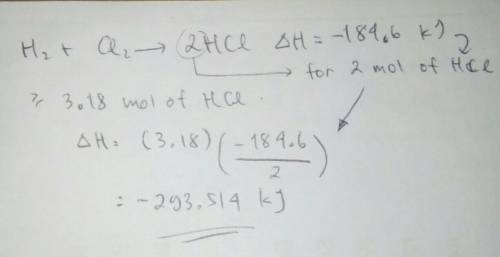Chemistry, 23.07.2019 04:00, maggiegoodenough62
How much heat is released during the formation of 3.18 mol hcl(g) in this reaction: h2(g)+cl2(g) → 2hcl(g) with a ∆h of -184.6 kj. express your answer in kj?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 21:50, SoccerAllStar2
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
Do you know the correct answer?
How much heat is released during the formation of 3.18 mol hcl(g) in this reaction: h2(g)+cl2(g) →...
Questions in other subjects:

Mathematics, 11.12.2020 02:10

Mathematics, 11.12.2020 02:10

Mathematics, 11.12.2020 02:10

Mathematics, 11.12.2020 02:10


English, 11.12.2020 02:10


History, 11.12.2020 02:10

Chemistry, 11.12.2020 02:10

Geography, 11.12.2020 02:10