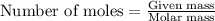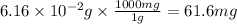Chemistry, 23.07.2019 10:00, coralstoner6793
Acid precipitation dripping on limestone produces carbon dioxide by the following reaction: caco3(s) + 2h+(aq) > ca(2+)(aq) + co2(g) + h2o (l) 15.5ml of co2 was produced at 25*c and 738.0 mmhg how man moles of co2 were produced? how many milligrams of caco3 were consumed?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, bakoeboo
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2


Chemistry, 21.06.2019 22:30, andaws21
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
Do you know the correct answer?
Acid precipitation dripping on limestone produces carbon dioxide by the following reaction: caco3(s...
Questions in other subjects:

Biology, 03.02.2020 09:01

World Languages, 03.02.2020 09:01

Geography, 03.02.2020 09:01

French, 03.02.2020 09:01




Mathematics, 03.02.2020 09:01



 and the mass of calcium carbonate is 61.6 mg
and the mass of calcium carbonate is 61.6 mg
![25^oC=[25+273]K=298K](/tpl/images/0123/0054/df1f6.png)
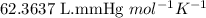

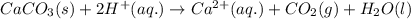
 of calcium carbonate
of calcium carbonate