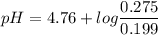Chemistry, 23.07.2019 12:30, teesoprettyy
Calculate the ph of 0.375 l of a 0.18 m acetic acid-0.29 m sodium acetate buffer after the addition of 0.0070 mol of hbr. assume that the volume remains constant.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1
Do you know the correct answer?
Calculate the ph of 0.375 l of a 0.18 m acetic acid-0.29 m sodium acetate buffer after the addition...
Questions in other subjects:

History, 29.04.2021 19:30

Mathematics, 29.04.2021 19:30

Mathematics, 29.04.2021 19:30


Mathematics, 29.04.2021 19:30


Biology, 29.04.2021 19:30


Chemistry, 29.04.2021 19:30

Spanish, 29.04.2021 19:30


![\displaystyle [H^+]=Ka\times\frac{mole\:weak\:acid}{mole\:salt\times valence}](/tpl/images/0123/4241/7d032.png)
![\displaystyle [OH^-]=Kb\times\frac{mole\:weak\:base}{mole\:salt\times valence}](/tpl/images/0123/4241/6b85e.png)
![\displaystyle pH=pKa+log\frac{[salt]}{[acid]}](/tpl/images/0123/4241/3aa97.png)