
Chemistry, 23.07.2019 13:30, nguyendavis17
The total number of atoms represented by the formula fe3(po4)2 is a. 11 b. 12 c. 13 d. 15

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, rome58
Lab reagent, hypothesis test. a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl. these six measurements are assumed to be an srs of all possible measurements from solution. they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution. carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Do you know the correct answer?
The total number of atoms represented by the formula fe3(po4)2 is a. 11 b. 12 c. 13 d. 15...
Questions in other subjects:

Mathematics, 06.10.2021 08:00


Mathematics, 06.10.2021 08:00

Mathematics, 06.10.2021 08:00

Mathematics, 06.10.2021 08:00

Mathematics, 06.10.2021 08:00

Mathematics, 06.10.2021 08:00

Mathematics, 06.10.2021 08:00


Biology, 06.10.2021 08:10


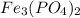
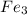
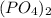
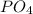 we will have 1 atom of P and 4 atoms of O. (When we dont have any number as subscript we have to assume that we have a "1")
we will have 1 atom of P and 4 atoms of O. (When we dont have any number as subscript we have to assume that we have a "1")



