
Chemistry, 23.07.2019 19:30, Jocelyn0925
Which of the following statements is true of combustion reactions? a. hydrogen gas (h2) must be one of the reactants b. water (h2o) is always a product c. energy is released by the reaction d. carbon monoxide (co2) is always produced

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 18:30, TaraC
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1
Do you know the correct answer?
Which of the following statements is true of combustion reactions? a. hydrogen gas (h2) must be one...
Questions in other subjects:

History, 15.06.2021 15:30

Arts, 15.06.2021 15:30

Mathematics, 15.06.2021 15:30

English, 15.06.2021 15:30


Mathematics, 15.06.2021 15:30

Mathematics, 15.06.2021 15:30




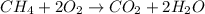
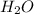 ) is always a product, is true of combustion reactions.
) is always a product, is true of combustion reactions.




