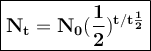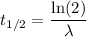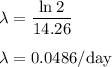Chemistry, 23.07.2019 21:00, jalenshayewilliams
The half-life of phosphorus-32 is 14.26 days. calculate its decay constant. express the decay constant numerically in inverse days.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:00, 2024cynthiatercero
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3
Do you know the correct answer?
The half-life of phosphorus-32 is 14.26 days. calculate its decay constant. express the decay consta...
Questions in other subjects:


Geography, 25.01.2020 13:31


Social Studies, 25.01.2020 13:31

English, 25.01.2020 13:31

Mathematics, 25.01.2020 13:31

Biology, 25.01.2020 13:31

Mathematics, 25.01.2020 13:31

Mathematics, 25.01.2020 13:31

Mathematics, 25.01.2020 13:31