
Chemistry, 24.07.2019 06:00, tshegofatso92
Astudent mixes a 10.0 ml sample of 1.0 m naoh(aq) with a 10.0 ml sample of 1.0 m hcl(aq) in a polystyrene container. the temperature of the solutions before mixing was 20.0°c. if the final temperature of the mixture is 26.0°c, what is the experimental value of dhrxn

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 04:31, 1Angel2Got3Brains
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
Do you know the correct answer?
Astudent mixes a 10.0 ml sample of 1.0 m naoh(aq) with a 10.0 ml sample of 1.0 m hcl(aq) in a polyst...
Questions in other subjects:

History, 13.01.2021 20:50



Mathematics, 13.01.2021 20:50

History, 13.01.2021 20:50


Mathematics, 13.01.2021 20:50

English, 13.01.2021 20:50

Mathematics, 13.01.2021 20:50


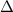
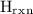 for the experiment will be 19.278 J.
for the experiment will be 19.278 J.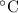
 Volume (L)
Volume (L)



